FLOWFLEX ANTIGEN RAPID TEST SELF TESTING - 1 PC
AED 25.00 – AED 20.00
VAT: AED 0 20% OFF
The Flowflex SARS-CoV-2 Rapid Antigen test is an immunochromatographic test for the qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen in nasopharyngeal swab (NP) samples from individuals suspected of COVID-19.
- Nasopharyngeal swab sample
- Results in 15 minutes
- Excellent performance compared to molecular methods.
- Sensitivity of 97.1%. 99.3% specificity
- Storage at room temperature
- Shelf life 2 years
- Pre-filled tubes of single-dose reagent
Availability: 0 In Stock
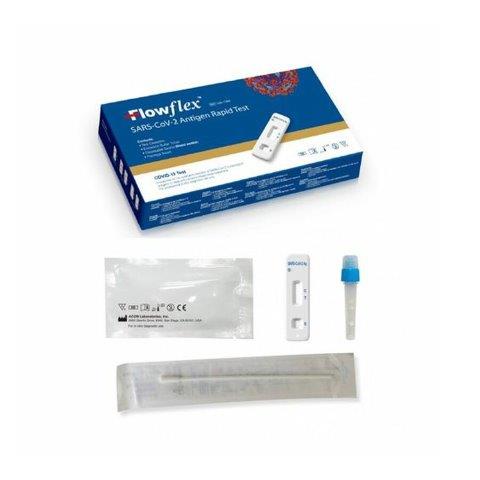
FLOWFLEX KIT COMPONENTS
- SARS-CoV-2 Antigen Test Cassette (Sealed Bag)
- Sterile swab
- Extraction tube
- Extraction solution
- Waste Bag
- Tube Support
- Instructions for use
HOW TO USE THE FLOWLEX ALLERGEN TEST?
Previous preparation for the test:
- Clear, clean and dry a flat surface.
- Check the content of the test kit
- Make sure it is not damaged or broken.
- Have a watch or stopwatch handy.
- Before taking the sample, blow your nose several times.
- Handwashing
Procedure for conducting the test:
- Remove the aluminum foil from the top of the extraction buffer tube.
- Insert the tube into the hole in the kit box (or place the tube in the tube holder).
- Open the swab packaging at the end of the toothpick. Caution: Do not touch the absorbent tip of the swab with your hands.
- Insert the entire absorbent tip of the swab into one of the nostrils. With gentle rotation, push the swab less than 2.5 cm from the edge of the nostril.
- Rotate the swab 5 times rubbing the inner walls of the nostril. Remove the swab and insert it into the other nostril. Repeat the previous step.
- Insert the swab into the tube and shake it for 30 seconds.
- Rotate the swab 5 times while pressing the side of the tube.
- Remove the swab while pressing the tube.
- Firmly screw the dropper cap onto the extraction buffer tube containing the sample. Mix the sample well by stirring or shaking the bottom of the tube.
- Gently squeeze the tube and dispense 4 drops of solution into the sample container.
- Read the result when the timer reaches 15-30 minutes. DO NOT read the result after 30 minutes.
- Then, dispose of the test and all the utensils that we have used for the test in the household waste container, following the protocol established by the health authorities. It should not be deposited at the SIGRE point of the pharmacy, or on public roads.
Interpretation of COVID-19 FLOWFLEX test results
- NEGATIVE: If within 15 to 30 minutes a colored line appears in the control zone (C) but no colored line is observed in the test zone (T), the test is valid and negative. A negative result does not exclude a SARS-CoV-2 viral infection and, if COVID-19 is suspected, it must be confirmed by molecular diagnostic methods.
- POSITIVE: If two colored lines appear within 15 to 30 minutes, one in the control zone (C) and the other in the test zone (T), the test is valid and positive. The result is considered positive no matter how faint the colored line of the test area (T) is. A positive result does not exclude a coinfection with other pathogens.
- INVALID: If no colored line appears in the control zone (C) within 15 to 30 minutes, the test is invalid. Repeat the test with a new test cassette.
PRECAUTIONS AND WARNINGS
- This test is designed for the rapid qualitative determination of the SARS-CoV-2 virus antigen in anterior nasal smears (front of the nose) in people with suspected COVID-19 during the first seven days after the onset of symptoms. The SARS-CoV-2 Rapid Antigen Test should not be used as the sole tool to diagnose or exclude a SARS-CoV-2 infection. Children under 18 must be assisted by an adult.
- The test must be used exclusively for the qualitative detection of the SARS-CoV-2 virus antigen in samples by an anteronasal smear (front part of the nose). The exact antigen concentration of the SARS-CoV-2 virus cannot be determined in the context of this test.
- Proper collection of samples is of vital importance. If the procedure is not followed, the test result may be inaccurate. Improper collection and storage or even freezing and thawing of the sample can lead to inaccurate results.
- If the viral load of the sample is below the detection limit of the test, the test may give a negative result.
- As in all diagnostic tests, the final clinical diagnosis should not be based on the result of a single test, but must be established by the doctor once all the clinical results and laboratory findings have been evaluated.
- Apart from SARS-CoV-2, a negative result does not exclude a viral infection and, in case of suspected COVID-19, it must be confirmed by molecular diagnostic methods.
- A positive result does not exclude a co-infection with other pathogens.
- The SARS-CoV-2 Rapid Antigen Test can detect both viable and non-viable SARS-CoV-2 material. The performance of the rapid SARS-CoV-2 antigen test is dependent on viral load and may not be correlated with other diagnostic methods used on the same sample.
- Users must analyze the samples as soon as possible after collection and, in any case, within two hours of collection.
- The sensitivity of nasal or oropharyngeal smears may be less than that of nasopharyngeal smears. It is recommended that the nasopharyngeal smear method be applied by healthcare personnel.
- Monoclonal antibodies that have undergone small amino acid changes in the target epitope region may not be able to detect the SARS-CoV-2 virus or may be less sensitive.
- The amount of antigen in a sample may decrease as the duration of the disease progresses. Specimens collected from the 5th to the 7th day of illness are more likely to be negative compared to an RT-PCR analysis.
- The validity of the SARS-CoV-2 rapid antigen test for the identification / confirmation of cultured tissue isolates has not been demonstrated and should not be used in this context.
- Cross-reactivity was evaluated by testing a panel of pathogens and related microorganisms that are likely to be present in the nasal cavity. Each organism and virus was tested in the absence or presence of the heat-inactivated SARS-CoV-2 virus at a low level of positivity.
- The kit can be stored at temperatures between 2 and 30 ° C.
- Do not freeze